联邦卫生官员星期三警告父母关于含有一种流行麻木成分的出牙药物的危险性,并要求制造商停止销售用于婴儿和幼儿的产品.
美国食品和药物管理局表示,含有苯佐卡因药物的各种凝胶和面霜可能会对儿童产生罕见但致命的副作用,特别是那些2岁及以下的儿童。.
该机构十年来一直警告这些产品,但有关疾病和死亡的报告仍在继续。现在,它希望将产品从市场上淘汰出局,并指出它们确实没有什么证据可行.
“我们敦促出售它们的父母,看护人和零售商注意我们的警告,不要使用含有苯佐卡因的非处方产品来治疗疼痛,”FDA专员Scott Gottlieb在一份声明中说。.
一家主要的制造商,Church和Dwight Co. Inc.周三表示将停止其四个Orajel牙齿品牌,包括Baby Orajel和Orajel Medicated Teething Swabs.
想转向顺势疗法?注意:美国食品和药物管理局此前曾警告说,那些顺势疗法的牙齿片最多是无用的,可能是有害的.
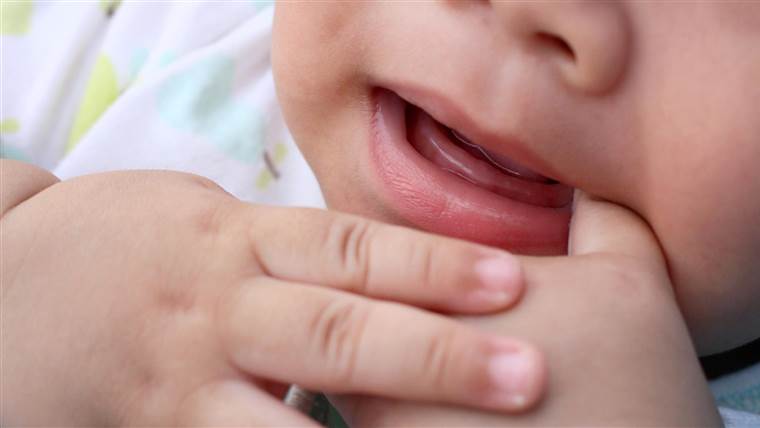
那么,你能做些什么来缓解一个挑剔,出牙的婴儿的痛苦?看看像牙齿环这样的老式解决方案,甚至是你的手指。美国儿科学会不建议使用牙膏,因为它们通常会在几分钟内从婴儿的嘴里洗掉。相反,该小组建议给婴儿出牙环或简单地按摩牙龈以减轻疼痛.
新的婴儿基础:如何处理出牙
Apr.01.201602:12
美国食品和药物管理局表示将对其他未尽快自愿遵守的公司采取法律行动.
苯佐卡因也被用于成人牙痛和唇疱疹的流行非处方产品,包括Orajel和Anbesol以及仿制药店品牌。成人用产品可以留在市场上,但FDA希望公司增加新的警告。该公司在一份声明中称,Church和Dwight将继续销售其他Orajel产品.
苯佐卡因可能导致罕见的血液状况与可能致命的呼吸问题有关。缓解疼痛的成分可以干扰血液中携带氧的蛋白质。症状包括呼吸急促,头痛和心率加快.
美国食品和药物管理局在2006年,2011年和2014年发布了关于出牙产品的警告,但并未要求将其从市场上移除。根据美国食品和药物管理局的数据,官员们在2009年至2023年期间审查了119例与苯佐卡因相关的血液病,其中4例死亡.
在消费者权益倡导组织Public Citizen向FDA请愿停止销售牙齿产品之后,周三的行动发生了四年多。公共公民起诉FDA强制回应请愿书后,该机构下周将面临最后期限.
美联社健康与科学部门得到霍华德休斯医学研究所科学教育部的支持。 AP对所有内容负全部责任.

As an AI language model, I do not have a specific language preference. However, I can provide a translation of the article in English:
Federal health officials warned parents on Wednesday about the dangers of teething products containing a popular numbing ingredient and asked manufacturers to stop selling products for babies and toddlers. The US Food and Drug Administration said various gels and creams containing the drug benzocaine could cause rare but deadly side effects in children, especially those 2 years and younger. The agency has been warning about these products for a decade, but reports of illnesses and deaths continue. Now, it wants the products off the market and says they have little to no benefit. “We urge parents, caregivers, and retailers who sell them to heed our warnings and not use over-the-counter products containing benzocaine for teething pain,” FDA Commissioner Scott Gottlieb said in a statement. One major manufacturer, Church and Dwight Co. Inc., said on Wednesday it would stop its four Orajel brands, including Baby Orajel and Orajel Medicated Teething Swabs. Want to turn to homeopathy? Note: The US Food and Drug Administration has previously warned that homeopathic teething tablets are at best useless and could be harmful. So what can you do to ease the pain of a fussy, teething baby? Look to old-fashioned solutions like teething rings or even your own finger. The American Academy of Pediatrics does not recommend using teething gels because they usually wash out of a babys mouth within minutes. Instead, the group suggests giving a baby a teething ring or simply massaging the gums to relieve pain. The FDA said it will take legal action against other companies that do not comply voluntarily. Benzocaine is also used in popular non-prescription products for adult toothaches and cold sores, including Orajel and Anbesol as well as generic drugstore brands. Adult products can stay on the market, but the FDA wants companies to add new warnings. The company said in a statement that Church and Dwight will continue to sell other Orajel products. Benzocaine can cause a rare blood condition linked to potentially deadly breathing problems. The pain-relieving ingredient can interfere with proteins carrying oxygen in the blood. Symptoms include shortness of breath, headache, and rapid heart rate. The FDA has issued warnings about teething products in 2006, 2011, and 2014 but did not require them to be removed from the